-
 Core | Water Machines
Core | Water Machines
Core Separations specializes in manufacturing a wide range of vital components utilized in CO2 extraction systems. As a prominent leader in the field of supercritical extraction, we maintain the highest standards of quality, safety, and functionality through our production methods and established practices. With more than 20 years of experience in working with supercritical fluids, our extensive knowledge positions us as experts in developing tailored systems for processing diverse natural products


Subcritical Water Processing
High-pressure and temperature water processing is instrumental in a range of research domains, allowing the extraction of valuable substances from herbs, seeds, and leaves. As a supplement to our environmentally friendly supercritical CO2 technology, subcritical water processing facilitates the extraction of more polar compounds. Notably, subcritical water also initiates controlled hydrolysis of proteins, eliminating the need for catalyst additives and making it a potent tool in natural product research.
Why not read about some of the most common applications below?
Static and Flow Operations
Static and Flow Operations
Given the wide range of applications for subcritical water, our systems cater to both reactive and extractive modes. For extracting substances from solid biomass, we employ automated back pressure control, enabling high-pressure, high-temperature continuous extractions. Similarly, using this technology, we provide static pressure holds controlled by our pump, aiding processes like protein hydrolysis. This versatility defines our Subcritical water system.
High temperature and Pressure
High Temperature and Pressure
Our systems are designed to reach notably high temperatures and pressures, making them versatile across a range of applications. With the capability to achieve 400°C at 500 bar, they are perfectly poised to further research in the subcritical water realm. By operating across such a broad spectrum of temperature and pressure, it enables the adjustment of water properties, facilitating variations in polarity via a reduced dielectric constant and altered ionic strength.
Safety
Safety
Given the elevated temperatures and pressures that the SFXW systems can reach, we prioritize safety at the heart of its design. All high-pressure, high-temperature tubing is shielded to prevent unintentional exposure to hazards. Moreover, both the vessel and pumps are equipped with safety mechanisms to prevent accidental damage to the equipment and ensure the safety of users.
Water System Options
Our systems are designed to be modular and upgradeable. This allows our customers to modify the systems to meet their research needs or processing requirements.
High Temperature
Furance
High Temperature Furance
For high-demand subcritical water processing requiring temperatures of up to 400 degrees Celsius, the Core | Water system incorporates a high-temperature clamshell furnace. This component is designed to effectively heat the extraction vessel.
Speciality MOC
Speciality MOC
By employing Inconel for the extraction vessel, heat exchangers, and high-pressure pipework, any areas potentially exposed to supercritical water are safeguarded. This measure helps minimize the possible corrosion caused by high-chloride biomass, which could otherwise compromise safety and reduce the system’s lifespan.
Flow Meters
Flow Meters
Water pumps fitted with flow meters possess the ability to precisely measure the water flow, guaranteeing meticulous regulation of water movement throughout the subcritical water process.
Unique Core Separations Features

Core | Pumps

SFX Control Software

Core | Vessels
Frequently Asked
Questions
Got any questions, just ask!
1. How does subcritical water extraction differ from CO2 Extraction?
2. How do CO2 and water systems differ?
3. What temperatures and pressures can we work at using subcritical water?
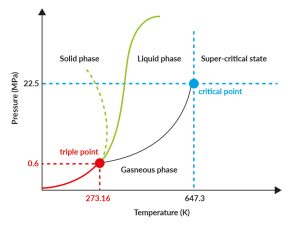
We can operate under a broad spectrum of pressures and temperatures within the subcritical region of water. As long as our operations are conducted below the supercritical threshold of water, which is 373 degrees Celsius and 220 bar, and above 100 degrees Celsius, we are indeed operating within the subcritical region of water.
Water phase diagram – Redraw (liquid phase = subcritical phase).
4. What else can subcritical water do other than extractions?
5. What is Hydrothermal Carbonisation?
Hydrothermal carbonization (HTC) is a thermochemical process that converts organic material, such as biomass, into a carbon-rich solid known as hydrochar. The mechanism of HTC involves several reactions including hydrolysis, dehydration, decarboxylation, and aromatization, leading to the formation of the hydrochar. The overall process reduces the oxygen and hydrogen content of the biomass while increasing the relative carbon content, thereby increasing its energy density.
Hydrochar generated through hydrothermal carbonization (HTC) exhibits coal-like properties and can serve as a solid fuel. Additionally, it possesses the potential for further refinement into valuable materials such as activated carbon. One significant advantage of hydrothermal carbonization is that the feedstock does not require prior drying before undergoing the process.
6. What is hydrothermal Liquefaction?
Hydrothermal liquefaction (HTL) is a thermochemical conversion process that involves the conversion of biomass or organic feedstock into a liquid product under high temperature and pressure conditions in the presence of water. During hydrothermal liquefaction, the biomass or organic material is typically mixed with water and subjected to temperatures ranging from 245 to 373 degrees Celsius and pressures ranging from 100 and 250 bar. Under these conditions, the biomass undergoes a series of chemical reactions, including hydrolysis, dehydration, decarboxylation, and hydrogenation.
The result of hydrothermal liquefaction is a liquid product known as bio-oil or biocrude, which has similarities to fossil crude oil. This bio-oil can be further refined and upgraded to produce transportation fuels, such as gasoline, diesel, and jet fuel. Additionally, other valuable co-products like solid biochar and aqueous phase can be obtained during the process.
Hydrothermal liquefaction offers several advantages, including the ability to process a wide range of feedstocks, including wet biomass and waste materials.
Why use Subcritical Water
Subcritical water processing is a favored method for extracting polar compounds like proteins and sugars from botanical sources. With its adjustable properties in the subcritical range (100-372°C), subcritical water can selectively extract various compounds. Moreover, it can hydrolyze proteins without needing any acid. These attributes offer distinctive advantages over traditional petrochemical-based alternatives.
Tuneable Ionic Strength
When water’s temperature and pressure rise, it transitions from a mainly polar nature to a more ionic one. This change enhances water’s reactivity, making it a potent solvent for many compounds that wouldn’t easily dissolve in cooler, low-pressure environments.
Tunable Polarity
At elevated pressures and temperatures, water’s density diminishes, reducing its polarity and improving its capacity to dissolve nonpolar compounds. On the other hand, at lower pressures and temperatures, water retains its polar nature, preserving its aptitude for dissolving polar substances.
Environmentally Responsible
Subcritical water extraction is environmentally friendly minimizing chemical use as the primary by-product is non-hazardous water, which can be treated and reused, supporting sustainability. Water, a renewable resource, combined with the system’s potential for minimal waste, makes subcritical water technologies a sustainable choice for various industrial processes.
Tunable Density
By modulating the pressure and temperature, we can fine-tune the properties of subcritical water to maximize its performance. The water’s density under subcritical conditions can be influenced by these adjustments which in turn greatly affects its solvating capability, viscosity, and diffusivity.
Learn how our systems work!
We strive to create systems that are user-friendly and efficient, ensuring a seamless experience from straightforward operations to low dead volume pipework. Our systems are designed to deliver exceptional selectivity in CO2 processing and maintain a high standard of extract quality.
Specification Downloads
Certification
-
 I
I
-
 I
I
-
 I
I
-
 I
I